Chronic heartburn isn’t just annoying-it can change the lining of your esophagus in ways that put you at risk for cancer. This isn’t speculation. It’s science. Every year, tens of thousands of people with long-term GERD develop Barrett’s esophagus, a condition where the normal tissue in the lower esophagus is replaced by intestinal-like cells. It’s not cancer. But it’s the most common precursor to esophageal adenocarcinoma, a deadly cancer with a five-year survival rate under 20% once symptoms appear.
What Exactly Is Barrett’s Esophagus?
Barrett’s esophagus happens because your esophagus is constantly exposed to stomach acid. Over time, the squamous cells that normally line the esophagus can’t handle the damage. So your body tries to protect itself by replacing them with columnar cells-cells that look more like those in the intestines. This is called intestinal metaplasia. It’s a survival tactic, but it’s also a warning sign.
This change doesn’t happen overnight. It usually takes at least 10 years of frequent, untreated acid reflux. The more often you have heartburn-especially if it’s three or more times a week-and the longer it’s been going on, the higher your risk. People who’ve had GERD for over 20 years are 40 times more likely to develop Barrett’s than those without chronic reflux.
It’s not random who gets it. Men are three times more likely than women. White men over 50 with a history of smoking or obesity are at the highest risk. In the U.S., about 5.6% of the population has Barrett’s esophagus. Among people with chronic GERD, that number jumps to 10-15%.
Why Screening Matters-Even If You Feel Fine
Here’s the hard truth: Barrett’s esophagus doesn’t cause symptoms of its own. If you’re having heartburn, that’s GERD. But if you’ve had heartburn for over a decade and suddenly stop feeling it, that doesn’t mean you’re better. It might mean your esophagus has changed so much that the nerves are damaged. You’re not feeling the acid anymore-but the damage is still happening.
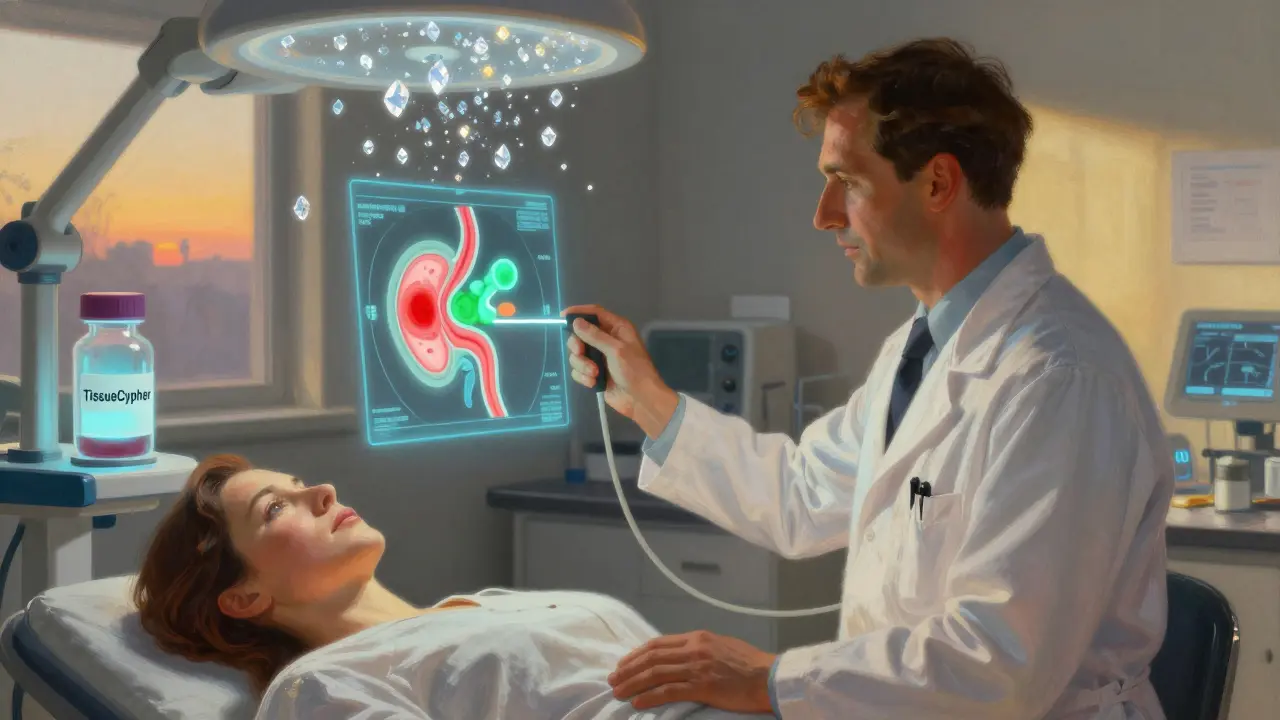
That’s why screening isn’t about symptoms. It’s about prevention. The only way to know if you have Barrett’s is through an upper endoscopy. During the procedure, a doctor looks for salmon-colored tissue above the stomach junction. But appearance alone isn’t enough. Biopsies are taken using the Seattle protocol: four tissue samples every 1 to 2 centimeters along the abnormal area. That’s usually 12 to 24 samples total. Without this, you could miss early signs of dysplasia.
Dysplasia means the cells are starting to look abnormal under the microscope. It’s graded as:
- Non-dysplastic Barrett’s esophagus (NDBE)-no precancerous changes
- Indefinite for dysplasia-unclear, needs repeat testing
- Low-grade dysplasia (LGD)-mild abnormalities
- High-grade dysplasia (HGD)-severe changes, almost cancer
High-grade dysplasia carries a 6-19% chance of turning into cancer each year. That’s why catching it early is life-saving.
Who Should Be Screened?
Not everyone with GERD needs an endoscopy. Guidelines are specific because screening is expensive and carries small risks. The American College of Gastroenterology recommends screening for:
- Men with chronic GERD (more than 5 years)
- Who have symptoms at least once a week
- And have at least one additional risk factor: age over 50, White race, smoking, obesity (BMI over 30), or a family history of Barrett’s or esophageal cancer
Women and younger men without other risk factors are generally not screened unless they have persistent symptoms despite treatment. Why? Because the overall risk is low, and the cost-benefit doesn’t favor routine screening in low-risk groups.
But here’s what patients don’t tell their doctors: many people assume their heartburn is normal. The Esophageal Cancer Action Network found that 68% of people with Barrett’s had symptoms for over five years before diagnosis. They thought it was just bad digestion. They didn’t connect chronic reflux to cancer risk. That delay is dangerous.
What Happens After Diagnosis?
If you’re diagnosed with non-dysplastic Barrett’s esophagus, you’re not in immediate danger. But you’re not out of the woods either. Surveillance is key. The standard is an endoscopy every 3 to 5 years. If your biopsies show low-grade dysplasia, you’ll need a second opinion from a pathologist who specializes in gastrointestinal disease. Then, you’ll likely have another endoscopy in 6 to 12 months.
High-grade dysplasia is different. Most doctors won’t wait. They’ll recommend endoscopic therapy right away. The gold standard is radiofrequency ablation (RFA). It uses heat to destroy the abnormal tissue. In clinical trials, RFA clears dysplasia in 90-98% of cases. Cryotherapy-freezing the tissue-is another option. Both are done during endoscopy, take less than an hour, and most people go home the same day.
One patient from Mayo Clinic, diagnosed with HGD after 15 years of heartburn, had RFA and was cancer-free after six months. He now has annual check-ups. His story isn’t rare. Thousands have had the same outcome.
Can You Prevent Progression?
Medication alone won’t cut it. Proton pump inhibitors (PPIs) like omeprazole or esomeprazole reduce acid and help symptoms. But studies show that even high-dose PPIs (40mg twice daily) fail to fully suppress acid in 30-45% of patients. You can feel better and still have ongoing damage.
Real protection means total acid control. That’s why some doctors recommend 24-hour esophageal pH monitoring to see if your meds are actually working. Lifestyle changes are just as important:
- Stop eating 3 hours before bed
- Elevate the head of your bed 6-8 inches
- Quit smoking
- Keep your BMI under 25
- Avoid alcohol, caffeine, chocolate, fatty foods, and spicy meals
Weight loss alone can reduce reflux episodes by 40%. One study showed that losing just 10% of body weight improved GERD symptoms as much as doubling the PPI dose.
What’s New in Screening and Treatment?
The field is changing fast. In 2021, Medicare started covering the TissueCypher test-a non-endoscopic blood and saliva test that analyzes molecular markers to predict cancer risk. It has a 96% negative predictive value. That means if the test says you’re low risk, you probably are. This could cut down unnecessary endoscopies by 40%.
In 2022, guidelines expanded RFA use to include all patients with confirmed low-grade dysplasia. Before, doctors often just monitored. Now, early intervention is standard because data shows 94% of LGD cases stay gone after five years with ablation.
Researchers are now testing DNA methylation markers to predict who will progress to cancer. A $2.4 million study running until 2026 in Texas is looking at whether these biomarkers can replace routine biopsies. If successful, we might one day screen with a simple blood test instead of an endoscope.
The Big Problem No One Talks About
Here’s the uncomfortable truth: 95% of people with Barrett’s esophagus will never develop cancer. But we don’t know which 5% will. So we screen everyone. And that means tens of thousands of people get endoscopies every year that don’t lead to cancer prevention. It’s expensive, stressful, and sometimes risky.
That’s why the future isn’t just about better tools-it’s about smarter targeting. We need to stop screening everyone with GERD. We need to find the people who are truly at risk. That’s where molecular testing and AI-driven risk models are heading.
Right now, if you’ve had chronic heartburn for over 10 years, especially if you’re a male over 50 with other risk factors, don’t wait for symptoms to worsen. Talk to your doctor about screening. It’s not about fear. It’s about control. You can’t stop GERD overnight. But you can stop it from turning into something worse.
Can Barrett’s esophagus go away on its own?
No, Barrett’s esophagus doesn’t reverse without treatment. The intestinal metaplasia that defines it is a permanent structural change in the esophagus lining. However, with effective acid suppression and endoscopic ablation, the abnormal tissue can be removed and replaced with normal squamous tissue. Studies show that after radiofrequency ablation, up to 90% of patients no longer have Barrett’s tissue visible on endoscopy. But without treatment, the condition remains and can progress.
Do proton pump inhibitors (PPIs) prevent cancer in Barrett’s esophagus?
PPIs help control symptoms and reduce acid exposure, but they don’t reliably prevent cancer. Multiple studies show that even high-dose PPIs fail to fully suppress acid in nearly half of patients. While some observational data suggest a possible protective effect, no clinical trial has proven that PPIs reduce the risk of esophageal cancer in Barrett’s patients. Complete acid control, confirmed by pH monitoring, is necessary-but even that doesn’t guarantee protection. Surveillance and ablation remain the only proven ways to reduce cancer risk.
Is Barrett’s esophagus hereditary?
There’s a genetic component. People with a first-degree relative (parent, sibling, child) who has Barrett’s esophagus or esophageal adenocarcinoma are at higher risk. Studies show a 2-3 times increased likelihood of developing Barrett’s if a close family member has it. While no single gene causes it, researchers believe inherited factors influence how the esophagus responds to acid damage and how quickly metaplasia develops. If you have a family history, talk to your doctor about earlier screening.
Can you have Barrett’s esophagus without GERD symptoms?
Yes. About 20-30% of people diagnosed with Barrett’s esophagus report no classic heartburn or regurgitation. This is called ‘silent GERD.’ The esophagus may have become desensitized due to long-term damage, or the reflux may be primarily non-acidic (bile or gas), which doesn’t trigger the same burning sensation. These patients often present with hoarseness, chronic cough, or throat clearing. Without screening, they’re at high risk of delayed diagnosis.
How often do you need an endoscopy if you have Barrett’s esophagus?
It depends on the level of dysplasia. For non-dysplastic Barrett’s, endoscopy is recommended every 3 to 5 years. If low-grade dysplasia is confirmed by an expert, surveillance is done every 6 to 12 months. High-grade dysplasia usually leads to treatment with ablation rather than continued surveillance. After successful ablation, follow-up endoscopies are done every 3 to 6 months for the first year, then annually if no recurrence is found. Always follow your doctor’s personalized plan-guidelines are general, but your risk profile is unique.

rasna saha
January 24, 2026 AT 21:56Wow, this was so eye-opening. I’ve had heartburn for years and just thought it was ‘normal’-never connected it to cancer risk. Thank you for laying it out so clearly. I’m booking an endoscopy next week.
Aurelie L.
January 25, 2026 AT 19:43So you’re telling me I need a scope just because I’m a fat white guy who smokes and likes pizza? Cool.
Kipper Pickens
January 27, 2026 AT 04:40Barrett’s esophagus is a classic example of metaplastic adaptation under chronic inflammatory pressure-specifically, IL-8 and NF-κB pathways drive the transdifferentiation of squamous to columnar epithelium via SOX2 and CDX2 upregulation. The real clinical dilemma isn’t detection-it’s risk stratification. We’re over-screening low-risk cohorts while under-resourcing molecular biomarkers like TissueCypher, which has a 96% NPV. The cost-benefit calculus needs recalibration.
shivam utkresth
January 27, 2026 AT 17:38Bro, I’ve been refluxing since college and thought it was just ‘spicy food karma.’ Now I’m scared I’m one biopsy away from a death sentence. 😅 But seriously-this post saved my life. I just canceled my weekend beer binge and started sleeping upright. Also, RFA sounds like sci-fi magic. 98% success? That’s wild.
Joanna Domżalska
January 28, 2026 AT 18:20So let me get this straight. You’re saying we should stick tubes down people’s throats because they ate too much tacos? And this is medicine? Or just fear-mongering with a stethoscope?
Faisal Mohamed
January 30, 2026 AT 15:08Imagine if we could just spit in a cup and know if we’re gonna die. 🧬🩸 TissueCypher is the future. Endoscopies are so 2010. Also, PPIs don’t fix anything-just mute the alarm. The house is still on fire. 🔥
Skye Kooyman
January 31, 2026 AT 08:52My dad had it. No symptoms. Died of cancer anyway. So yeah. Screen.
James Nicoll
January 31, 2026 AT 14:54So we’re doing all this to prevent a cancer that only affects 5% of people with Barrett’s? That’s like locking your door because someone, somewhere, might steal your left sock. 😏
Uche Okoro
February 1, 2026 AT 17:58Chronic acid exposure induces epigenetic modifications in esophageal progenitor cells, particularly hypermethylation of tumor suppressor genes like CDKN2A and RUNX3. This is not mere metaplasia-it’s pre-neoplastic reprogramming. The current surveillance paradigm is reactive, not predictive. We need longitudinal methylome profiling integrated with AI-driven phenotyping. Otherwise, we’re just chasing ghosts with a scope.
Ashley Porter
February 3, 2026 AT 06:57Just read this after my endo last month. NDBE. My doc said ‘keep taking your PPIs and lose weight.’ I did both. Lost 22 lbs. No heartburn in 8 months. Still getting scoped every 3 years. Worth it.
John Wippler
February 3, 2026 AT 10:52Here’s the thing no one says out loud: we’re treating Barrett’s like it’s a ticking bomb. But what if it’s more like a slow leak? We’ve spent billions scanning people who’ll never get cancer-while ignoring the real villain: chronic inflammation from processed food, stress, and sleep deprivation. The fix isn’t just a scope or a laser. It’s a lifestyle revolution. And we’re too scared to have that conversation.
My uncle had HGD. Got RFA. Now he hikes, meditates, and eats kale smoothies. He didn’t ‘beat cancer.’ He changed his life. And yeah, the scope sucked. But the alternative? Worse.
We don’t need more tests. We need more people who care enough to change. The body heals when you stop punishing it. Acid reflux isn’t a snack problem. It’s a life problem. And we’re treating it like a coupon.
So yeah. Get screened if you’re high risk. But also-stop eating at 11pm. Stop smoking. Move your body. Sleep like your life depends on it. Because it does.
And if you’re reading this and you’ve ignored heartburn for 10 years? Don’t wait for silence. That’s not peace. That’s your body giving up.