Category: Medications - Page 3
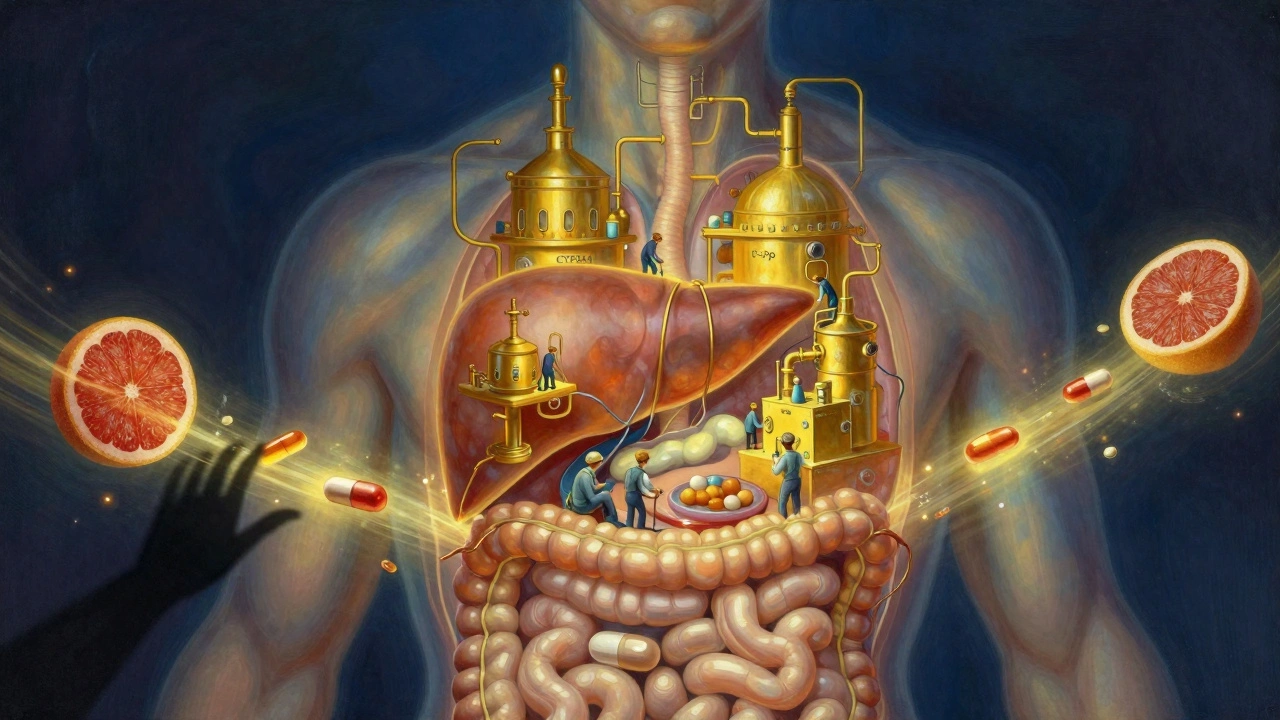
Antiviral Medications and CYP3A4/P-gp Interactions: What You Need to Know
Antiviral medications like those for HIV and hepatitis C can dangerously interact with common drugs through CYP3A4 and P-glycoprotein. Learn how to avoid life-threatening side effects with simple checks and safer treatment options.
Immunogenicity in Biosimilars: Why Immune Responses May Differ from Reference Biologics
Biosimilars are not exact copies of biologics-tiny structural differences can trigger immune responses. Learn why immunogenicity varies between reference drugs and biosimilars, and what it means for patients.
Chinese Generic Production: Manufacturing and Quality Concerns in Global Pharma
Chinese manufacturers produce 80% of the world's generic drug ingredients, but quality issues, outdated tech, and supply chain risks threaten global medicine safety. Here's what you need to know.
FDA Orange Book: Where to Find Patent Expiration Dates for Generic Drug Entry
Learn how to find patent expiration dates in the FDA Orange Book to predict when generic drugs will become available. Includes step-by-step search tips, common pitfalls, and how to verify data.
Extended Use Dates: FDA Allowances During Drug Shortages
The FDA extends expiration dates for critical drugs during shortages to ensure patient access when supply is low. These extensions are data-driven, lot-specific, and only granted for life-saving medications with no safe alternatives.
Vitamin E and Warfarin: What You Need to Know About the Bleeding Risk
Vitamin E supplements can increase bleeding risk when taken with warfarin, especially at doses above 400 IU daily. Learn what the evidence says, which doses are dangerous, and how to stay safe while on anticoagulant therapy.
Authorized Generics: How Brand Drug Companies Respond to Patent Expiration
Authorized generics are brand-name drugs sold without the brand label, offering identical ingredients at lower prices. They’re a strategic response to patent expiration, helping manufacturers keep market share while lowering costs for patients.
Pregnancy and Lactation Labeling Rule (PLLR): How to Read FDA Drug Safety Info
The FDA's Pregnancy and Lactation Labeling Rule (PLLR) replaced outdated letter categories with clear, evidence-based safety info for pregnant and nursing women. Learn how to read the new labels and make informed medication decisions.
How Pharmacists Prevent Prescription Medication Errors Every Day
Pharmacists prevent hundreds of thousands of medication errors each year by catching mistakes in prescriptions before they reach patients. Learn how they use technology, training, and clinical judgment to keep people safe.
Oral Thrush from Medications: How to Treat and Prevent Antifungal Side Effects
Oral thrush is a common side effect of steroids, antibiotics, and immunosuppressants. Learn how nystatin and fluconazole treat it, why prevention matters, and how to avoid recurrence with simple daily habits.